Patient specific multisite pacing of diseased human hearts
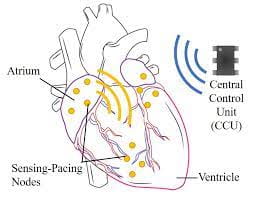
Cardiovascular disease is the number one cause of death worldwide. For example, heart failure is a significant source of mortality within the U.S; it is responsible for 1% of all emergency room presentations and contributes to one in every nine deaths. A significant proportion of heart failure patients have concurrent conduction system disease, which will lead to their eventual death. Cardiac resynchronization has proven to be a useful therapy for improving cardiac function as well as reducing mortality in some patients. However, a significant number of patients fail to respond to resynchronization therapy, often due to inadequate pacemaker lead placement. There currently exists no pacemaker system that provides the “potential” benefits of multisite temporally and spatially precise pacing for resynchronization.
To reach the audacious goal of eliminating cardiovascular diseases, new technologies must be developed that will monitor the diseased heart with unprecedented temporal and spatial precision and will manage the pacing of the heart to restore a healthy function of the heart. The proposed system will process data recorded from multiple sites and will generate a pacing therapy specific to the patient in real-time.
The proposed project revisits the core foundation of pacemakers to develop multisite temporally and spatially precise pacing for resynchronization. The PIs will build a robust, well-annotated database of intracardiac electrograms (IEGM) from multiple cardiac sites. The focus will be on identifying challenging cases that are clinically difficult to differentiate and, thus, stand to reap the greatest benefit of being able to direct overall algorithm development. This information will be added to the associated metadata file. Data will be collected from a minimum of 150 patients with at least 50 patients from each identified pathophysiology. The PIs will identify the pathology of each patient based on data from multiple intracardiac recording sites. The proposed machine-learning pipeline explores the representation of time series using wavelets and then learns transformations of multiple time series using the Lie group framework. Finally the pipeline clusters time series using clustering of the transformations to identify the right pathology for the specific patient. In addition, the PIs will explore implementation as an application specific integrated circuit (ASIC) that will be implanted subcutaneously to continuously process intracardiac multisite recordings as well as to generate temporally and spatially precise pacing patterns.
Behnaam Aazhang, PhD
J.S. Abercrombie Professor, Electrical and Computer Engineering Director, Rice Neuroengineering Initiative (NEI), Rice University